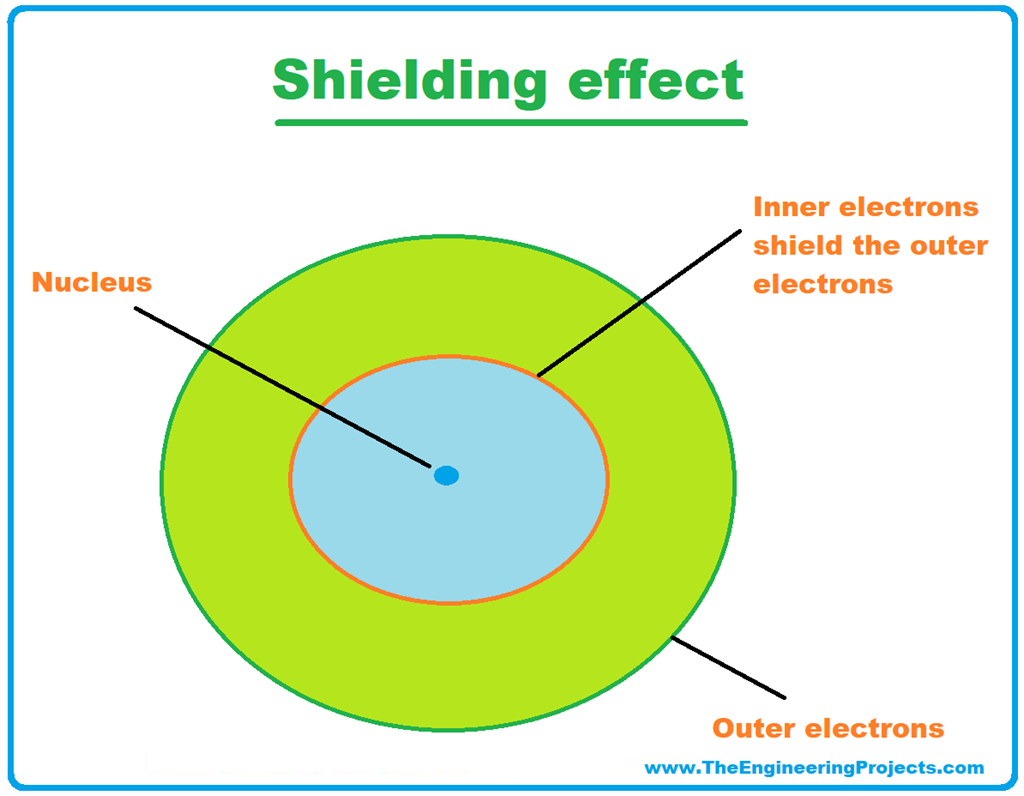
Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period . The trend depends on shell and subshell, but generally z* increases across a period. A higher efective nuclear charge causes greater. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. Across a period, efective nuclear charge increases as electron shielding remains constant. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. When moving to the right of a period, the number of. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an.
from www.theengineeringprojects.com
A higher efective nuclear charge causes greater. When moving to the right of a period, the number of. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. The trend depends on shell and subshell, but generally z* increases across a period. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. Across a period, efective nuclear charge increases as electron shielding remains constant.
Periodic Table of Elements Definition, Groups & Trends The
Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period A higher efective nuclear charge causes greater. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. Across a period, efective nuclear charge increases as electron shielding remains constant. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. The trend depends on shell and subshell, but generally z* increases across a period. A higher efective nuclear charge causes greater. When moving to the right of a period, the number of.
From perso.numericable.fr
The electrons in the inner shells shield the electrons in the outer Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. The trend depends on shell and subshell, but generally z* increases across a period. Across a period, efective nuclear charge increases as electron shielding remains constant. When moving to the right of a period, the. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The trend depends on shell and subshell, but generally z* increases across a period. When moving to the right of a period, the number of. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From socratic.org
How are shielding effect and atomic radius related? Socratic Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. The trend depends on shell and subshell, but generally z* increases across a period. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. A higher efective nuclear charge causes greater. When moving to the right of a period, the. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period A higher efective nuclear charge causes greater. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. Across a period, efective nuclear charge increases as electron shielding remains constant. The trend depends on shell and subshell, but generally z* increases across a period. When moving. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.slideserve.com
PPT Unit 2 20142015 PowerPoint Presentation, free download ID Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. The trend depends on shell and subshell, but generally z* increases across a period. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. When moving to the right. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period When moving to the right of a period, the number of. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. Across a period, efective nuclear. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period Across a period, efective nuclear charge increases as electron shielding remains constant. A higher efective nuclear charge causes greater. When moving to the right of a period, the number of. The trend depends on shell and subshell, but generally z* increases across a period. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From mungfali.com
What Is Shielding Effect Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period Across a period, efective nuclear charge increases as electron shielding remains constant. When moving to the right of a period, the number of. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. A higher efective nuclear charge causes greater. The trend depends on shell. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From dokumen.tips
(PPTX) Periodic Trends. Describe factors that affect electron position Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period Across a period, efective nuclear charge increases as electron shielding remains constant. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. In. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. Across a period, efective nuclear charge increases as electron shielding remains constant. When moving to the right of a period, the number of. The trend depends on shell and subshell, but generally z* increases across a period. The. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.slideshare.net
Periodic trends Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. A higher efective nuclear charge causes greater. The trend depends on shell and subshell, but generally z* increases across a period. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.chem.fsu.edu
Electron Configurations Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period The d orbital electrons are poorly shielding compared to the s and p orbital electrons so effective nuclear charge increases more rapidly. The trend depends on shell and subshell, but generally z* increases across a period. A higher efective nuclear charge causes greater. Across a period, efective nuclear charge increases as electron shielding remains constant. The only way for ionization. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. The trend depends on shell and subshell, but generally z* increases across a period. Across a period, efective nuclear charge increases as electron shielding remains constant. The d orbital electrons are poorly shielding compared to the s and p orbital electrons. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From surfguppy.com
What is Electronegativity? Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period Across a period, efective nuclear charge increases as electron shielding remains constant. When moving to the right of a period, the number of. A higher efective nuclear charge causes greater. The trend depends on shell and subshell, but generally z* increases across a period. The d orbital electrons are poorly shielding compared to the s and p orbital electrons so. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From www.slideserve.com
PPT Periodic Groups and Trends PowerPoint Presentation, free download Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period Across a period, efective nuclear charge increases as electron shielding remains constant. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on. The trend depends on shell and subshell, but generally z* increases across a period. The d orbital electrons are poorly shielding compared to. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.
From sciencenotes.org
Electron Affinity Trend and Definition Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an. When moving to the right of a period, the number of. The trend depends on shell and subshell, but generally z* increases across a period. The only way for ionization energy to increase across a period is if the only the. Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period.